Mechanical ventilation
In medicine, mechanical ventilation is a method to mechanically assist or replace spontaneous breathing.
This may involve a machine called a ventilator or the breathing may be assisted by a physician or other suitable person compressing a bag or set of bellows. Traditionally divided into negative-pressure ventilation, where air is essentially sucked into the lungs, or positive pressure ventilation, where air (or another gas mix) is pushed into the trachea.
It can be used as a short term measure, for example during an operation or critical illness (often in the setting of an intensive care unit). It may be used at home or in a nursing or rehabilitation institution if patients have chronic illnesses that require long-term ventilatory assistance.
Owing to the anatomy of the human pharynx, larynx, and esophagus and the circumstances for which ventilation is required then additional measures are often required to "secure" the airway during positive pressure ventilation to allow unimpeded passage of air into the trachea and avoid air passing into the esophagus and stomach. Commonly this is by insertion of a tube into the trachea which provides a clear route for the air. This can be either an endotracheal tube, inserted through the natural openings of mouth or nose or a tracheostomy inserted through an artificial opening in the neck. In other circumstances simple airway maneuvres, an oropharyngeal airway or laryngeal mask airway may be employed. If the patient is able to protect their own airway such as in non-invasive ventilation or negative-pressure ventilation then no airway adjunct may be needed.
Mechanical ventilation is often a life-saving intervention, but carries many potential complications including pneumothorax, airway injury, alveolar damage, and ventilator-associated pneumonia..
In many healthcare systems prolonged ventilation as part of intensive care is a limited resource (in that there are only so many patients that can receive care at any given moment). It is used to support a single failing organ system (the lungs) and cannot reverse any underlying disease process (such as terminal cancer). For this reason there can be (occasionally difficult) decisions to be made about whether it is suitable to commence someone on mechanical ventilation. Equally many ethical issues surround the decision to discontinue mechanical ventilation.
History
The Roman physician Galen may have been the first to describe mechanical ventilation: "If you take a dead animal and blow air through its larynx [through a reed], you will fill its bronchi and watch its lungs attain the greatest distention."[1] Vesalius too describes ventilation by inserting a reed or cane into the trachea of animals[2]. In 1908 George Poe demonstrated his mechanical respirator by asphyxiating dogs and seemingly bringing them back to life.[3]
Negative pressure machines
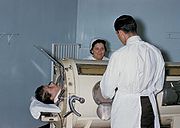
The iron lung, also known as the Drinker and Shaw tank, was developed in 1929 and was one of the first negative-pressure machines used for long-term ventilation. It was refined and used in the 20th century largely as a result of the polio epidemic that struck the world in the 1940s. The machine is effectively a large elongated tank, which encases the patient up to the neck. The neck is sealed with a rubber gasket so that the patient's face (and airway) are exposed to the room air.
While the exchange of oxygen and carbon dioxide between the bloodstream and the pulmonary airspace works by diffusion and requires no external work, air must be moved into and out of the lungs to make it available to the gas exchange process. In spontaneous breathing, a negative pressure is created in the pleural cavity by the muscles of respiration, and the resulting gradient between the atmospheric pressure and the pressure inside the thorax generates a flow of air.
In the iron lung by means of a pump, the air is withdrawn mechanically to produce a vacuum inside the tank, thus creating negative pressure. This negative pressure leads to expansion of the chest, which causes a decrease in intrapulmonary pressure, and increases flow of ambient air into the lungs. As the vacuum is released, the pressure inside the tank equalizes to that of the ambient pressure, and the elastic coil of the chest and lungs leads to passive exhalation. However, when the vacuum is created, the abdomen also expands along with the lung, cutting off venous flow back to the heart, leading to pooling of venous blood in the lower extremities. There are large portholes for nurse or home assistant access. The patients can talk and eat normally, and can see the world through a well-placed series of mirrors. Some could remain in these iron lungs for years at a time quite successfully.
Today, negative pressure mechanical ventilators are still in use, notably with the Polio Wing Hospitals in England such as St. Thomas' (by Westminster in London) and the John Radcliffe in Oxford. The prominent device used is a smaller device known as the cuirass. The cuirass is a shell-like unit, creating negative pressure only to the chest using a combination of a fitting shell and a soft bladder. Its main use is in patients with neuromuscular disorders who have some residual muscular function. However, it was prone to falling off and caused severe chafing and skin damage and was not used as a long term device. In recent years this device has re-surfaced as a modern polycarbonate shell with multiple seals and a high pressure oscillation pump in order to carry out biphasic cuirass ventilation.
Positive pressure machines

The design of the modern positive-pressure ventilators were mainly based on technical developments by the military during World War II to supply oxygen to fighter pilots in high altitude. Such ventilators replaced the iron lungs as safe endotracheal tubes with high volume/low pressure cuffs were developed. The popularity of positive-pressure ventilators rose during the polio epidemic in the 1950s in Scandinavia and the United States and was the beginning of modern ventilation therapy. Positive pressure through manual supply of 50% oxygen through a tracheostomy tube led to a reduced mortality rate among patients with polio and respiratory paralysis. However, because of the sheer amount of man-power required for such manual intervention, mechanical positive-pressure ventilators became increasingly popular.
Positive-pressure ventilators work by increasing the patient's airway pressure through an endotracheal or tracheostomy tube. The positive pressure allows air to flow into the airway until the ventilator breath is terminated. Subsequently, the airway pressure drops to zero, and the elastic recoil of the chest wall and lungs push the tidal volume -- the breath—out through passive exhalation.
This is an example of a neonatal (infant) ventilator.
Indications for use
Mechanical ventilation is indicated when the patient's spontaneous ventilation is inadequate to maintain life. It is also indicated as prophylaxis for imminent collapse of other physiologic functions, or ineffective gas exchange in the lungs. Because mechanical ventilation only serves to provide assistance for breathing and does not cure a disease, the patient's underlying condition should be correctable and should resolve over time. In addition, other factors must be taken into consideration because mechanical ventilation is not without its complications (see below)
Common medical indications for use include:
- Acute lung injury (including ARDS, trauma)
- Apnea with respiratory arrest, including cases from intoxication
- Chronic obstructive pulmonary disease (COPD)
- Acute respiratory acidosis with partial pressure of carbon dioxide (pCO2) > 50 mmHg and pH < 7.25, which may be due to paralysis of the diaphragm due to Guillain-Barré syndrome, Myasthenia Gravis, spinal cord injury, or the effect of anaesthetic and muscle relaxant drugs
- Increased work of breathing as evidenced by significant tachypnea, retractions, and other physical signs of respiratory distress
- Hypoxemia with arterial partial pressure of oxygen (PaO2) with supplemental fraction of inspired oxygen (FiO2) < 55 mm Hg
- Hypotension including sepsis, shock, congestive heart failure
- Neurological diseases such as Muscular Dystrophy Amyotrophic Lateral Sclerosis
Types of ventilators
Ventilation can be delivered via:
- Hand-controlled ventilation such as:
 SMART BAG MO Bag-Valve-Mask Resuscitator
SMART BAG MO Bag-Valve-Mask Resuscitator- Bag valve mask
- Continuous-flow or Anaesthesia (or T-piece) bag
- A mechanical ventilator. Types of mechanical ventilators include:
- Transport ventilators. These ventilators are small, more rugged, and can be powered pneumatically or via AC or DC power sources.
- ICU ventilators. These ventilators are larger and usually run on AC power (though virtually all contain a battery to facilitate intra-facility transport and as a back-up in the event of a power failure). This style of ventilator often provides greater control of a wide variety of ventilation parameters (such as inspiratory rise time). Many ICU ventilators also incorporate graphics to provide visual feedback of each breath.
- NICU ventilators. Designed with the preterm neonate in mind, these are a specialized subset of ICU ventilators which are designed to deliver the smaller, more precise volumes and pressures required to ventilate these patients.
- PAP ventilators. these ventilators are specifically designed for non-invasive ventilation. this includes ventilators for use at home, in order to treat sleep apnea.
Modes of ventilation
Conventional ventilation
The modes of ventilation can be thought of as classifications based on how to control the ventilator breath. Traditionally ventilators were classified based on how they determined when to stop giving a breath. The three traditional categories of ventilators are listed below. As microprocessor technology is incorporated into ventilator design, the distinction among these types has become less clear as ventilators may use combinations of all of these modes as well as flow-sensing, which controls the ventilator breath based on the flow-rate of gas versus a specific volume, pressure, or time.
Breath termination
Modes of ventilation are classified by the means that they determine the inspired breath is complete. This is sensed by either pressure or volume.
- Volume ventilation - A predetermined tidal volume (Vt) is set for the patient and is delivered with each inspiration. The amount of pressure necessary to deliver this volume will fluctuate from breath to breath based on the resistance and compliance of the patient and ventilator circuit. If the tidal volume is set at 500ml, the ventilator will continue to inspire gas until it reaches its goal. Upon completion of the inspired volume, the ventilator will open a valve allowing the patient to passively exhale.
- Pressure ventilation - A predetermined peak inspiratory pressure (PIP) is determined based on the patient's condition and pathophysiology. The ventilator will flow gas into the patient until this set pressure is reached. Upon reaching the preset PIP, the ventilator allows for passive exhalation. Caution and close observation must be given in this mode due to potential for either hypoventilation or hyperventilation because the tidal volume is variable.
Several manufactures have incorporated features from both of theses modes in an attempt to accommodate patients needs.
These modes are flow-variable, volume-targeted, pressure-regulated, time-limited modes (for example, pressure regulated volume control - PRVC). This means that instead of providing an exact tidal volume each breath, a target volume is set and the ventilator will vary the inspiratory flow at each breath to achieve the target volume at the lowest possible peak pressure. The inspiratory time (Ti) limits the length of the inspiratory cycle and therefore the I:E ratio. Pressure regulated modes such as PRVC or Auto-flow (Draeger) can most easily be thought of as turning a volume mode into a pressure mode with the added benefit of maintaining more control over tidal volume than with strictly pressure-control.
Breath initiation
The other method of classifying mechanical ventilation is based on how to determine when to start giving a breath. Similar to the termination classification noted above, microprocessor control has resulted in a myriad of hybrid modes that combine features of the traditional classifications. Note that most of the timing initiation classifications below can be combined with any of the termination classifications listed above.
- Assist Control (AC). In this mode the ventilator provides a mechanical breath with either a pre-set tidal volume or peak pressure every time the patient initiates a breath. Traditional assist-control used only a pre-set tidal volume—when a preset peak pressure is used this is also sometimes termed Intermittent Positive Pressure Ventilation or IPPV. However, the initiation timing is the same—both provide a ventilator breath with every patient effort. In most ventilators a back-up minimum breath rate can be set in the event that the patient becomes apnoeic. Although a maximum rate is not usually set, an alarm can be set if the ventilator cycles too frequently. This can alert that the patient is tachypneic or that the ventilator may be auto-cycling (a problem that results when the ventilator interprets fluctuations in the circuit due to the last breath termination as a new breath initiation attempt).
- Synchronized Intermittent Mandatory Ventilation (SIMV). In this mode the ventilator provides a pre-set mechanical breath (pressure or volume limited) every specified number of seconds (determined by dividing the respiratory rate into 60 seconds - thus a respiratory rate of 12 results in a 5 second cycle time). Within that cycle time the ventilator waits for the patient to initiate a breath using either a pressure or flow sensor. When the ventilator senses the first patient breathing attempt within the cycle, it delivers the preset ventilator breath. If the patient fails to initiate a breath, the ventilator delivers a mechanical breath at the end of the breath cycle. Additional spontaneous breaths after the first one within the breath cycle do not trigger another SIMV breath. However, SIMV may be combined with pressure support (see below). SIMV is frequently employed as a method of decreasing ventilatory support (weaning) by turning down the rate, which requires the patient to take additional breaths beyond the SIMV triggered breath.
- Controlled Mechanical Ventilation (CMV). In this mode the ventilator provides a mechanical breath on a preset timing. Patient respiratory efforts are ignored. This is generally uncomfortable for children and adults who are conscious and is usually only used in an unconscious patient. It may also be used in infants who often quickly adapt their breathing pattern to the ventilator timing.
- Pressure Support Ventilation (PSV). When a patient attempts to breathe spontaneously through an endotracheal tube, the narrowed diameter of the airway results in higher resistance to airflow, and thus a higher work of breathing. PSV was developed as a method to decrease the work of breathing in-between ventilator mandated breaths by providing an elevated pressure triggered by spontaneous breathing that "supports" ventilation during inspiration. Thus, for example, SIMV might be combined with PSV so that additional breaths beyond the SIMV programmed breaths are supported. However, while the SIMV mandated breaths have a preset volume or peak pressure, the PSV breaths are designed to cut short when the inspiratory flow reaches a percentage of the peak inspiratory flow (e.g. 10-25%). New generation of ventilators provides user-adjustable inspiration cycling off threshold, and some even are equipped with automatic inspiration cycling off threshold function. This helps the patient ventilator synchrony[4]. The peak pressure set for the PSV breaths is usually a lower pressure than that set for the full ventilator mandated breath. PSV can be also be used as an independent mode.
- Continuous Positive Airway Pressure (CPAP). A continuous level of elevated pressure is provided through the patient circuit to maintain adequate oxygenation, decrease the work of breathing, and decrease the work of the heart (such as in left-sided heart failure — CHF). Note that no cycling of ventilator pressures occurs and the patient must initiate all breaths. In addition, no additional pressure above the CPAP pressure is provided during those breaths. CPAP may be used invasively through an endotracheal tube or tracheostomy or non-invasively with a face mask or nasal prongs.
- Positive end-expiratory pressure (PEEP) is functionally the same as CPAP, but refers to the use of an elevated pressure during the expiratory phase of the ventilatory cycle. After delivery of the set amount of breath by the ventilator, the patient then exhales passively. The volume of gas remaining in the lung after a normal expiration is termed the functional residual capacity (FRC). The FRC is primarily determined by the elastic qualities of the lung and the chest wall. In many lung diseases, the FRC is reduced due to collapse of the unstable alveoli, leading to a decreased surface area for gas exchange and intrapulmonary shunting (see above), with wasted oxygen inspired. Adding PEEP can reduce the work of breathing (at low levels) and help preserve FRC.
APRV (Airway Pressure Release Ventilation)
APRV begins from an elevated baseline (called Phigh or measured high pressure) and achieves tidal ventilation with a brief release of the Phigh. This brief release allows CO2 removal through passive exhalation secondary to elastic recoil. The exhalation time (Tlow) is shortened to usually less than one second to prevent alveolar derecruitment and collapse - it is essentially CPAP with a brief release.
Ever increasing empirical evidence and clinical experience is showing that APRV is the primary mode of choice when ventilating a patient with ARDS or ALI (Acute Lung Injury).
Advantages to APRV ventilation include: decreased airway pressures, decreased minute ventilation, decreased dead-space ventilation, promotion of spontaneous breathing, almost 24 hour a day alveolar recruitment, decreased use of sedation, near elimination of neuromuscular blockade, optimized arterial blood gas results, mechanical restoration of FRC (functional residual capacity), a positive effect on cardiac output (due to the negative inflection from the elevated baseline with each spontaneous breath), increased organ and tissue perfusion, potential for increased urine output due to increased renal perfusion.
A patient with ARDS on average spends 8 to 11 days on a mechanical ventilator; APRV may reduce this time significantly and therefore reduce the incidence of VAP (ventilator acquired pneumonia), a risk that increases with each hour an intubated patient spends on the ventilator (VAP rate is 100% at 100 days on the vent) and carries with it a near 50% mortality rate. So, hospitals that are reporting a 0% incidence of VAP, may be improperly coding or improperly reporting.
* A controlled clinical trial testing APRV against the current ARDSNet protocol must be initiated.
High Frequency Ventilation (HFV)

High-Frequency Ventilation refers to ventilation that occurs at rates significantly above that found in natural breathing (as high as 240-900 "breaths" per minute). Within the category of high-frequency ventilation, the three principal types are high-frequency jet ventilation (HFJV), high-frequency flow interruption (HFFI), and high-frequency oscillatory ventilation (HFOV).
High Frequency Jet Ventilation employs a endotracheal tube adaptor in place for the normal 15 mm ET tube adaptor. A high pressure ‘’jet’’ of gas flows out of the adaptor and into the airway. This jet of gas occurs for a very brief duration, about 0.02 seconds, and at high frequency: 4-11 hertz. Tidal volumes ≤ 1 ml/Kg are used during HFJV. This combination of small tidal volumes delivered for very short periods of time create the lowest possible distal airway and alveolar pressures produced by a mechanical ventilator. Exhalation is passive. Jet ventilators utilize various I:E ratios--between 1:1.1 and 1:12-- to help achieve optimal exhalation. Conventional mechanical breaths are sometimes used to aid in reinflating the lung. Optimal PEEP is used to maintain alveolar inflation and promote ventilation-to-perfusion matching. Jet ventilation has been shown to reduce ventilator induced lung injury by as much as 20%.
"HFFI" operates similarly to a conventional ventilator, providing increased circuit pressure during the inspiratory phase and dropping back to PEEP during the expiratory phase.
In "HFOV" the pressure wave is driven by an electromagnetically controlled diaphragm similar to a loudspeaker. Because this can rapidly change the volume in the circuit, HFOV can produce a pressure that is lower than ambient pressure during the expiratory phase. This is sometimes called "active" expiration. In both types of high-frequency ventilation the pressure wave that is generated at the ventilator is markedly attenuated by passage down the endotracheal tube and the major conducting airways. This helps protect the alveoli from volutrauma that occurs with traditional positive pressure ventilation. Although the alveoli are kept at a relatively constant volume, similar to CPAP, other mechanisms of gas exchange allow ventilation (the removal of CO2) to occur without tidal volume exchange. Ventilation in HFO[[File:]]V is a function of frequency, amplitude, and I:E ratio and is best described graphically as the area under the curve of an oscillatory cycle. Amplitude is analogous to tidal volume in conventional ventilation; larger amplitudes remove more CO2. Seemingly paradoxical, lower frequencies remove more CO2 in HFOV whereas in conventional ventilation the opposite is true. As frequency decreases, there is less attenuation of the pressure wave transmitted to the alveoli. This results in increased mixing of gas and thus ventilation. I-time is set as a percentage of total time (usually 33%). Amplitude is a function of power and is subject to variability due to changes in compliance or resistance. Therefore, power requirements may vary significantly during treatment and from patient to patient. Patient characteristics and ventilator settings determine whether PaCO2 changes may be more sensitive to amplitude or frequency manipulation. In HFOV, mean airway pressure (MAP) is delivered via a continuous flow through the patient circuit which passes through a variable restriction valve (mushroom valve) on the expiratory limb. Increasing the flow through the circuit and/or increasing the pressure in the mushroom valve increases MAP. The MAP in HFOV functions similarly to PEEP in conventional ventilation in that it provides the pressure for alveolar recruitment.
Non-invasive ventilation (Non-invasive Positive Pressure Ventilation or NIPPV)
This refers to all modalities that assist ventilation without the use of an endotracheal tube. Non-invasive ventilation is primarily aimed at minimizing patient discomfort and the complications associated with invasive ventilation. It is often used in cardiac disease, exacerbations of chronic pulmonary disease, sleep apnea, and neuromuscular diseases. Non-invasive ventilation refers only to the patient interface and not the mode of ventilation used; modes may include spontaneous or control modes and may be either pressure or volume modes.
Some commonly used modes of NIPPV include:
- Continuous positive airway pressure (CPAP).
- Bi-level Positive Airway Pressure (BIPAP). Pressures alternate between Inspiratory Positive Airway Pressure (IPAP) and a lower Expiratory Positive Airway Pressure (EPAP), triggered by patient effort. On many such devices, backup rates may be set, which deliver IPAP pressures even if patients fail to initiate a breath.(Wheatley 2000 et all)
- Intermittent positive pressure ventilation (IPPV) via mouthpiece or mask
Proportional Assist Ventilation (PAV)
Proportional Assist Ventilation (PAV) is a form of synchronised ventilator support based upon the Equation of Motion in which the ventilator generates pressure in proportion to the instantaneous patient effort. Unlike other modes of partial support, there is no target flow, tidal volume or pressure. PAV’s objective is to allow the patient to attain ventilation and breathing pattern his ventilatory control system desires. The main operational advantages of PAV are automatic synchrony with inspiratory efforts, exhalation and adaptability to change in ventilatory demand.
Proportional Assist Ventilation Plus — PAV+ (Puritan Bennett – 840 ventilator range, Proportional Pressure Support — PPS (Drager Evita series)and Respironics BiPAP Vision PAV , are commercially available implementations of PAV which automatically amplify the patient's own spontaneous effort to breathe by increasing airway pressure during inspiration proportionally to a set amplification factor.
In PAV+, the level of amplification, thus the level of work of breathing, is set through a single setting (%support) and the pressure applied is continuously and automatically adjusted based on measures (including automatic assessment of Elastance and Resistance) taken throughout the inspiratory cycle to maintain an appropriate level of support.
Adaptive Support Ventilation (ASV)
Adaptive Support Ventilation (ASV) is a positive pressure mode of mechanical ventilation that is closed-loop controlled. In this mode, the frequency and tidal volume of breaths of a patient on the ventilator are automatically adjusted based on the patient’s requirements. The lung mechanics data are used to adjust the depth and rate of breaths to minimize the work rate of breathing. In the ASV mode, every breath is synchronized with patient effort if such an effort exists, and otherwise, full mechanical ventilation is provided to the patient.
ASV technology was originally described as one of the embodiments of US Patent No. 4986268.[5] In this invention, a modified version of an equation derived in physiology in 1950 [6] to minimize the work rate of breathing in man, was used for the first time to find the optimum frequency of mechanical ventilation. The rationale was to make the patient's breathing pattern comfortable and natural within safe limits, and thereby stimulate spontaneous breathing and reduce the weaning time. A prototype of the system was built by the inventor in late 1980s. The inventor is Dr. Fleur T. Tehrani who is a university professor in the US. Shortly after the Patent was issued in 1991, Hamilton Medical, a ventilator manufacturing company, contacted the inventor and discussed marketing the technology with her. Some years later, Hamilton Medical marketed this closed-loop technique under license of this Patent as ASV.
Since the issuance of the Patent, a number of articles have been published by the inventor and her colleagues that are related to the invention, and some of them describe further advancements of the closed-loop techniques presented in the Patent.[7]
Neurally Adjusted Ventilatory Assist (NAVA)
Neurally Adjusted Ventilatory Assist (NAVA) is a unique positive pressure mode to mechanical ventilation based on neural respiratory output, in connections with invasive and non-invasive NAVA.
The act of taking a breath is controlled by the respiratory center of the brain, which decides the characteristics of each breath, timing and size. The respiratory center sends a signal along the phrenic nerve, excites the diaphragm muscle cells, leading to muscle contraction and descent of the diaphragm dome. As a result, the pressure in the airway drops, causing an inflow of air into the lungs.
With NAVA, the electrical activity of the diaphragm (Edi) is captured, fed to the ventilator and used to assist the patient's breathing in synchrony with and in proportion to the patients own efforts, regardless of patient category or size. As the work of the ventilator and the diaphragm is controlled by the same signal, coupling between the diaphragm and the SERVO-i ventilator is synchronized simultaneously. Reference: New method permits neural control of mechanical ventilation
Choosing amongst ventilator modes
Assist-control mode minimizes patient effort by providing full mechanical support with every breath. This is often the initial mode chosen for adults because it provides the greatest degree of support. In patients with less severe respiratory failure, other modes such as SIMV may be appropriate. Assist-control mode should not be used in those patients with a potential for respiratory alkalosis, in which the patient has an increased respiratory drive. Such hyperventilation and hypocapnia (decreased systemic carbon dioxide due to hyperventilation) usually occurs in patients with end-stage liver disease, hyperventilatory sepsis, and head trauma. Respiratory alkalosis will be evident from the initial arterial blood gas obtained, and the mode of ventilation can then be changed if so desired.
Positive End Expiratory Pressure may or may not be employed to prevent atelectasis in adult patients. It is almost always used for pediatric and neonatal patients due to their increased tendency for atelectasis.
High frequency oscillation is used most frequently in neonates, but is also used as an always alternative mode in adults with severe ARDS.
Pressure Regulated Volume Control is another option.
Initial ventilator settings
The following are general guidelines that may need to be modified for the individual patient.
Tidal volume, rate, and pressures
- For adult patients and older children
- tidal volume (Tv) is calculated in milliliters per kilogram. Traditionally 10 ml/kg was used but has been shown to cause barotrauma, or injury to the lung by overextension, so 6 to 8 ml/kg is now common practice in ICU. Hence a patient weighing 70 kg would get a TV of 420–480 ml. In adults a rate of 12 strokes per minute is generally used.
- with acute respiratory distress syndrome (ARDS) a tidal volume of 6–8 ml/kg is used with a rate of 10–12 per minute. This reduced tidal volume allows for minimal volutrauma but may result in an elevated pCO2 (due to the relative decreased oxygen delivered) but this elevation does not need to be corrected (termed permissive hypercapnia)
- For infants and younger children
- without existing lung disease—a tidal volume of 4–8 ml/kg to be delivered at a rate of 30–35 breaths per minute
- with RDS—decrease tidal volume and increase respiratory rate sufficient to maintain pCO2 between 45 and 55. Allowing higher pCO2 (sometimes called permissive hypercapnia) may help prevent ventilator induced lung injury
As the amount of tidal volume increases, the pressure required to administer that volume is increased. This pressure is known as the peak airway pressure. If the peak airway pressure is persistently above 45 cmH2O (4.4 kPa) for adults, the risk of barotrauma is increased (see below) and efforts should be made to try to reduce the peak airway pressure. In infants and children it is unclear what level of peak pressure may cause damage. In general, keeping peak pressures below 30 cmH2O (2.9 kPa) is desirable.
Monitoring for barotrauma can also involve measuring the plateau pressure, which is the pressure after the delivery of the tidal volume but before the patient is allowed to exhale. Normal breathing pattern involves inspiration, then expiration. The ventilator is programmed so that after delivery of the tidal volume (inspiration), the patient is not allowed to exhale for a half a second. Therefore, pressure must be maintained in order to prevent exhalation, and this pressure is the plateau pressure. Barotrauma is minimized when the plateau pressure is maintained < 30–35 cmH2O.
Sighs
An adult patient breathing spontaneously will usually sigh about 6–8 times per hour to prevent microatelectasis, and this has led some to propose that ventilators should deliver 1½–2 times the amount of the preset tidal volume 6–8 times per hour to account for the sighs. However, such high quantity of volume delivery requires very high peak pressure that predisposes to barotrauma. Currently, accounting for sighs is not recommended if the patient is receiving 10-12 mL/kg or is on PEEP. If the tidal volume used is lower, the sigh adjustment can be used, as long as the peak and plateau pressures are acceptable.
Sighs are not generally used with ventilation of infants and young children.
Initial FiO2
Because the mechanical ventilator is responsible for assisting in a patient's breathing, it must then also be able to deliver an adequate amount of oxygen in each breath. The FiO2 stands for fraction of inspired oxygen, which means the percent of oxygen in each breath that is inspired. (Note that normal room air has ~21% oxygen content). In adult patients who can tolerate higher levels of oxygen for a period of time, the initial FiO2 may be set at 100% until arterial blood gases can document adequate oxygenation. An FiO2 of 100% for an extended period of time can be dangerous, but it can protect against hypoxemia from unexpected intubation problems. For infants, and especially in premature infants, avoiding high levels of FiO2 (>60%) is important.
Positive end-expiratory pressure (PEEP)
PEEP is an adjuvant to the mode of ventilation used to help maintain functional residual capacity (FRC). At the end of expiration, the PEEP exerts pressure to oppose passive emptying of the lung and to keep the airway pressure above the atmospheric pressure. The presence of PEEP opens up collapsed or unstable alveoli and increases the FRC and surface area for gas exchange, thus reducing the size of the shunt. For example, if a large shunt is found to exist based on the estimation from 100% FiO2 (see above), then PEEP can be considered and the FiO2 can be lowered (< 60%) in order to maintain an adequate PaO2, thus reducing the risk of oxygen toxicity.
In addition to treating a shunt, PEEP may also be useful to decrease the work of breathing. In pulmonary physiology, compliance is a measure of the "stiffness" of the lung and chest wall. The mathematical formula for compliance (C) equals change in volume divided by change in pressure. The higher the compliance, the more easily the lungs will inflate in response to positive pressure. An underinflated lung will have low compliance and PEEP will improve this initially by increasing the FRC, since the partially inflated lung takes less energy to inflate further. Excessive PEEP can however produce overinflation, which will again decrease compliance. Therefore it is important to maintain an adequate, but not excessive FRC.
Indications. PEEP can cause significant haemodynamic consequences through decreasing venous return to the right heart and decreasing right ventricular function. As such, it should be judiciously used and is indicated for adults in two circumstances.
- If a PaO2 of 60 mmHg cannot be achieved with a FiO2 of 60%
- If the initial shunt estimation is greater than 25%
If used, PEEP is usually set with the minimal positive pressure to maintain an adequate PaO2 with a safe FiO2. As PEEP increases intrathoracic pressure, there can be a resulting decrease in venous return and decrease in cardiac output. A PEEP of less than 10 cmH2O (1 kPa) is usually safe in adults if intravascular volume depletion is absent. Lower levels are used for pediatric patients. Older literature recommended routine placement of a Swan-Ganz catheter if the amount of PEEP used is greater than 10 cmH2 for hemodynamic monitoring. More recent literature has failed to find outcome benefits with routine PA catheterisation when compared to simple central venous pressure monitoring.[8] If cardiac output measurement is required, minimally invasive techniques, such as oesophageal doppler monitoring or arterial waveform contour monitoring may be sufficient alternatives.[9][10] PEEP should be withdrawn from a patient until adequate PaO2 can be maintained with a FiO2 < 40%. When withdrawing, it is decreased through 1–2 cmH2O decrements while monitoring haemoglobin-oxygen saturations. Any unacceptable haemoglobin-oxygen saturation should prompt reinstitution of the last PEEP level that maintained good saturation.
Positioning
Prone (face down) positioning has been used in patients with ARDS and severe hypoxemia. It improves FRC, drainage of secretions, and ventilation-perfusion matching (efficiency of gas exchange). It may improve oxygenation in > 50% of patients, but no survival benefit has been documented.
Sedation and Paralysis
Most intubated patients receive intravenous sedation through a continuous infusion or scheduled dosing to help with anxiety or psychological stress. Sedation also helps the patient tolerate the constant irritation of the endotracheal tube in their mouth, pharynx and trachea. Without some form of sedation and analgesia, it is common for patients to "fight" the ventilator. This fighting increases work of breathing and may cause further lung injury. Daily interruption of sedation is commonly helpful to the patient for reorientation and appropriate weaning. These interruptions are frequently described as "sedation vacations" and have been shown to reduce the time patients stay on mechanical ventilation.[11]
It is not uncommon for patients on a mechanical ventilator to be given a muscle relaxant or paralytic to aid in ventilation. These "neuromuscular blockades" prevent skeletal muscle from contracting and thereby stop all patient movement including respiratory efforts. These types of pharmaceutical agents must always be given in conjunction with sedation as the effects of the paralytics is not only uncomfortable but would cause significant psychological stress and anxiety.
Prophylaxis
- To protect against ventilator-associated pneumonia, patients' beds are often elevated to about 30°.
- Deep vein thrombosis prophylaxis with heparin or sequential compression device is important in older children and adults.
- A histamine receptor (H2) blocker or proton-pump inhibitor may be used to prevent gastrointestinal bleeding, which has been associated with mechanical ventilation
Modification of settings
In adults when 100% FiO2 is used initially, it is easy to calculate the next FiO2 to be used and easy to estimate the shunt fraction. The estimated shunt fraction refers to the amount of oxygen not being absorbed into the circulation. In normal physiology, gas exchange (oxygen/carbon dioxide) occurs at the level of the alveoli in the lungs. The existence of a shunt refers to any process that hinders this gas exchange, leading to wasted oxygen inspired and the flow of un-oxygenated blood back to the left heart (which ultimately supplies the rest of the body with unoxygenated blood).
When using 100% FiO2, the degree of shunting is estimated by subtracting the measured PaO2 (from an arterial blood gas) from 700 mmHg. For each difference of 100 mmHg, the shunt is 5%. A shunt of more than 25% should prompt a search for the cause of this hypoxemia, such as mainstem intubation or pneumothorax, and should be treated accordingly. If such complications are not present, other causes must be sought after, and PEEP should be used to treat this intrapulmonary shunt. Other such causes of a shunt include:
- Alveolar collapse from major atelectasis
- Alveolar collection of material other than gas, such as pus from pneumonia, water and protein from acute respiratory distress syndrome, water from congestive heart failure, or blood from haemorrhage
When to withdraw mechanical ventilation
Withdrawal from mechanical ventilation—also known as weaning—should not be delayed unnecessarily, nor should it be done prematurely. Patients should have their ventilation considered for withdrawal if they are able to support their own ventilation and oxygenation, and this should be assessed continuously. There are several objective parameters to look for when considering withdrawal, but there is no specific criteria that generalizes to all patients.
Trials of spontaneous breathing have been shown to accurately predict the success of spontaneous breathing. (Yang K, Tobin MJ. A prospective study of indexes predicting the outcome of weaning from mechanical ventilation. N Engl J Med 1991;324:1445–1450).
Connection to ventilators
There are various procedures and mechanical devices that provide protection against airway collapse, air leakage, and aspiration:
- Face mask - In resuscitation and for minor procedures under anaesthesia, a face mask is often sufficient to achieve a seal against air leakage. Airway patency of the unconscious patient is maintained either by manipulation of the jaw or by the use of nasopharyngeal or oropharyngeal airway. These are designed to provide a passage of air to the pharynx through the nose or mouth, respectively. Poorly fitted masks often cause nasal bridge ulcers, a problem for some patients. Face masks are also used for non-invasive ventilation in conscious patients. A full face mask does not, however, provide protection against aspiration.
- Laryngeal mask airway - The laryngeal mask airway (LMA) causes less pain and coughing than a tracheal tube. However, unlike tracheal tubes it does not seal against aspiration, making careful individualised evaluation and patient selection mandatory.
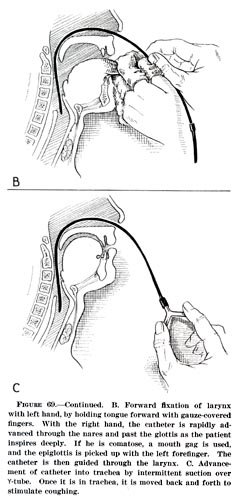
- Tracheal intubation is often performed for mechanical ventilation of hours to weeks duration. A tube is inserted through the nose (nasotracheal intubation) or mouth (orotracheal intubation) and advanced into the trachea. In most cases tubes with inflatable cuffs are used for protection against leakage and aspiration. Intubation with a cuffed tube is thought to provide the best protection against aspiration. Tracheal tubes inevitably cause pain and coughing. Therefore, unless a patient is unconscious or anaesthetized for other reasons, sedative drugs are usually given to provide tolerance of the tube. Other disadvantages of tracheal intubation include damage to the mucosal lining of the nasopharynx or oropharynx and subglottic stenosis.
- Esophageal obturator airway - sometimes used by emergency medical technicians and basic EMS providers not trained to intubate. It is a tube which is inserted into the esophagus, past the epiglottis. Once it is inserted, a bladder at the tip of the airway is inflated, to block ("obturate") the esophagus, and oxygen is delivered through a series of holes in the side of the tube which is then forced into the lungs.
- Cricothyrotomy - Patients who require emergency airway management, in whom tracheal intubation has been unsuccessful, may require an airway inserted through a surgical opening in the cricothyroid membrane. This is similar to a tracheostomy but a cricothyrotomy is reserved for emergency access.[1]
- Tracheostomy - When patients require mechanical ventilation for several weeks, a tracheostomy may provide the most suitable access to the trachea. A tracheostomy is a surgically created passage into the trachea. Tracheostomy tubes are well tolerated and often do not necessitate any use of sedative drugs. Tracheostomy tubes may be inserted early during treatment in patients with pre-existing severe respiratory disease, or in any patient who is expected to be difficult to wean from mechanical ventilation, i.e., patients who have little muscular reserve.
- Mouthpiece - Less common interface, does not provide protection against aspiration. There are lipseal mouthpieces with flanges to help hold them in place if patient is unable.
Terminology
Terminology used in the field of mechanical ventilation and respiratory support:
- APRV Airway pressure release ventilation
- ASB Assisted spontaneous breathing—also ASV = assisted spontaneous ventilation
- ASV Adaptive support ventilation—a patented technology—closed-loop mechanical respiration, a further development of MMV. Can also stand for assisted spontaneous ventilation.
- ATC Automatic tube compensation
- Automode Automode
- BIPAP Bilevel Positive Airway Pressure
- CMV Continuous mandatory ventilation
- CPAP Continuous positive airway pressure
- CPPV Continuous positive pressure ventilation
- EPAP Expiratory positive airway pressure
- HFV High frequency ventilation
- HFFI High frequency flow interruption
- HFJV High frequency jet ventilation
- HFOV High frequency oscillatory ventilation
- HFPPV High frequency positive pressure ventilation
- ILV Independent lung ventilation—separate sides positive pressure ventilation.
- IPAP Inspiratory positive airway pressure
- IPPV Intermittent positive pressure ventilation
- IRV Inversed ratio ventilation— mechanical ventilation with switched respiration phases/time rate.
- LFPPV Low frequency positive pressure ventilation
- MMV Mandatory minute volume
- NAVA Neurally Adjusted Ventilatory Assist
- NIF Negative inspiratory force—amount of force generated by a patient against a closed valve; greater than 20 cmH2O indicates an adequately strong diaphragm.
- NIV Non-invasive ventilation
- PAP Positive airway pressure
- PAV and PAV+ Proportional assist ventilation and proportional assist ventilation plus
- P/F ratio Ratio of PaO2 off an ABG and FiO2 off the ventilator. P/F < 200 indicates ARDS, P/F < 300 indicates ALI
- PCMV (P-CMV) Pressure controlled mandatory ventilation
- PCV Pressure controlled ventilation or PC Pressure control—pressure-controlled, fully mechanical ventilation.
- PEEP Positive end-expiratory pressure
- PNPV Positive negative pressure ventilation—switching pressure mechanical ventilation
- PPS Proportional pressure support
- PRVC Pressure regulated volume controlled ventilation
- PSV Pressure Support Ventilation or PS—supported spontaneous respiration, see also ASB.
- RSBI Rapid shallow breathing index—ratio of breath rate divided by the tidal volume. RSBI<105 declares a patient can be extubated and maintain themselves. Also indicates patient has a good chance of staying extubated.[12]
- (S) IMV (Synchronized) intermittent mandatory ventilation
- S-CPPV Synchronized continuous positive pressure ventilation
- S-IPPV Synchronized intermittent positive pressure ventilation
- TNI Therapy with nasal insufflation—nasal high-flow mechanical ventilation for respiration support.
- VCMV (V-CMV) Volume controlled mandatory ventilation
- VCV Volume controlled ventilation or VC, Volume Control—volume-controlled, fully mechanical ventilation.
- VS Volume Support
- ZAP Zero airway pressure—spontaneous respiration under atmospheric pressure.
See also
- Medical ventilator
References
- ↑ Colice, Gene L (2006). "Historical Perspective on the Development of Mechanical Ventilation". In Martin J Tobin. Principles & Practice of Mechanical Ventilation (2 ed.). New York: McGraw-Hill. ISBN 978-0071447676.
- ↑ Chamberlain D (2003) "Never quite there: A tale of resuscitation medicine" Clinical Medicine, Journal of the Royal College of Physicians' 3 6:573-577
- ↑ "Smother Small Dog To See it Revived. Successful Demonstration of an Artificial Respiration Machine Cheered in Brooklyn. Women in the Audience, But Most of Those Present Were Physicians. The Dog, Gathered in from the Street, Wagged Its Tail.". New York Times. May 29, 1908, Friday. http://en.wikipedia.org/wiki/Image:Poe_1908May29.gif. Retrieved 2007-12-25. "An audience, composed of about thirty men and three or four women, most of the men being physicians, attended a demonstration of Prof. George Poe's machine for producing artificial respiration in the library of the Kings County Medical Society, at 1,313 Bedford Avenue, Brooklyn, last night, under the auspices of the First Legion of the Red Cross Society."
- ↑ Expiratory Asynchrony. By Hong-Lin Du and Yoshitsugu Yamada, Respiratory Care Clinics of North America 2005;11:265-280.
- ↑ Tehrani, F. T., “Method and Apparatus for Controlling an Artificial Resirator,” US Patent No. 4986268, issued Jan. 22, 1991.
- ↑ Otis, A. B., Fenn, W. O., Rahn, H. "Mechanics of Breathing in Man." Journal of Applied Physiology, Volume 2: 592-607, 1950.
- ↑ See other references under further readings
- ↑ Shah, MR et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA. 2005 October 5;294(13):1664-70. PMID: 16204666
- ↑ Vallee F, et al. Stroke output variations calculated by oesophageal Doppler is a reliable predictor of fluid response. Intensive Care Med. 2005 Oct;31(10):1388-93. Epub 2005 August 19. PMID: 16132887
- ↑ Uchino S, et al. Pulmonary artery catheter versus pulse contour analysis: a prospective epidemiological study. Crit Care. 2006 December 14;10(6):R174 [Epub ahead of print] PMID: 17169160
- ↑ Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. New England Journal of Medicine. May 18 2000;342(20):1471–1477.
- ↑ Critical Care | Full text | Rapid shallow breathing index—a key predictor for noninvasive ventilation
Further reading
- Irwin R, Rippe J, "Intensive care medicine", 5th Edition, 2003 Lippincott Williams & Wilkins
- Irwin R, Rippe J, "Procedures and Techniques in Intensive care medicine", 3rd Edition, 2003 Lippincott Williams & Wilkins
- Marino P, "The ICU Book", 3rd Edition, 2006 Lippincott Williams & Wilkins
- Tehrani, F. T., “Automatic Control of an Artificial Respirator,” Proceedings of the International Conference of IEEE Engineering in Medicine & Biology Society, Volume 13: 1738-9, November 1991.
- Tehrani, F. T., Roum, J. H., "Closed-loop Control of Artificial Respiration," Proceedings of WESCON , pp 253–8, October 1996.
- Tehrani, F. T., "A Dual Automatic Control System for Ventilatory Treatment of Premature Infants," Proceedings of the World Multiconference on systemics, Cybernetics and Informatics (SCI 99), Volume 8:232-6, August 1999.
- Tehrani, F. T., "The Combined Effects of Closed-Loop Mechanical Ventilation and Automatic Control of Oxygen on Ventilatory Therapy: A Simulation Study," Proceedings of IASTED International Conference on Applied Modelling & Simulation, Volume 1:395-9, September 1999.
- Tehrani, F. T., "Automatic Control of Mechanical Ventilation and the Inspired Fraction of Oxygen in the Premature Infant: A Simulation Study," Proceedings of the International Conference of IEEE Engineering in Medicine & Biology Society,Volume 21:339, October 1999.
- Lo, T., Tehrani, F. T., Rogers, M., Lum, M., Malinowski, T., Afuwape, S., Terry, M., Grundl, B., “A Dual Closed-Loop Controller for Mechanical Ventilation,” American Journal of Respiratory and Critical Care Medicine, Volume 165: A376, April 2002.
- Tehrani, F. T., Rogers, M., Lo, T., Malinowski, T., Afuwape, S., Lum, M., Grundl, B., Terry, M., “A Dual Closed-Loop Control System for Mechanical Ventilation,” Journal of Clinical Monitoring and Computing, Volume 18, No. 2: 111-29, April 2004.
- Tehrani, F. T., “The origin of adaptive support ventilation,” the International Journal of Artificial Organs, Volume 28, No. 10: 1051-2, 2005.
- Tehrani, F. T., “A New Decision Support System for Mechanical Ventilation,” Proceedings of the International Conference of IEEE Engineering in Medicine & Biology Society, Volume 29: 3569-3572, August 2007 .
- Tehrani, F. T., Roum, J. H., “FLEX: A New Computerized System for Mechanical Ventilation,” Journal of Clinical Monitoring and Computing, Volume 22: 121- 130, 2008.
External links
- e-Medicine, article on mechanical ventilation along with technical information.
- Dr. Bach, a doctor experienced in use of noninvasive ventilation for patients with neuromuscular diseases (note: site is written by a third-party).
- International Ventilator Users Network (IVUN), Resource of information for users of home mechanical ventilation.
- Read more about NAVA, Neurally Adjusted Ventilatory Assist
- Experience of Neurally Adjusted Ventilatory Assist
|
||||||||||||||||||||||||||||||||||